Describe How Ionic Compounds Form Crystals
Why ionic compounds are formed. A good example is a sugar crystal which contains sucrose molecules.
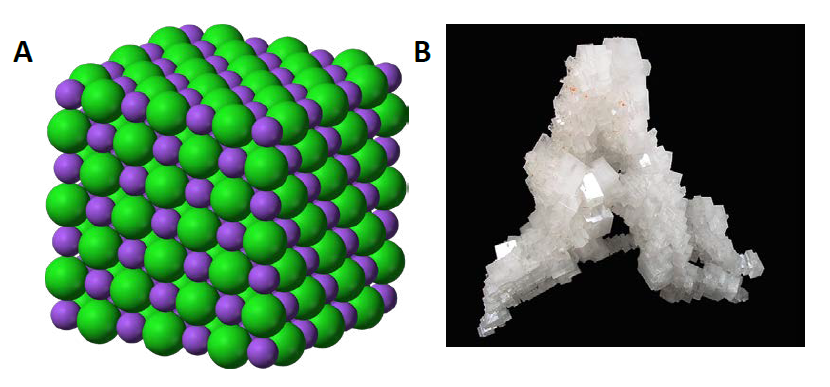
Ch104 Chapter 3 Ions And Ionic Compounds Chemistry
Well theres a couple of reasons.
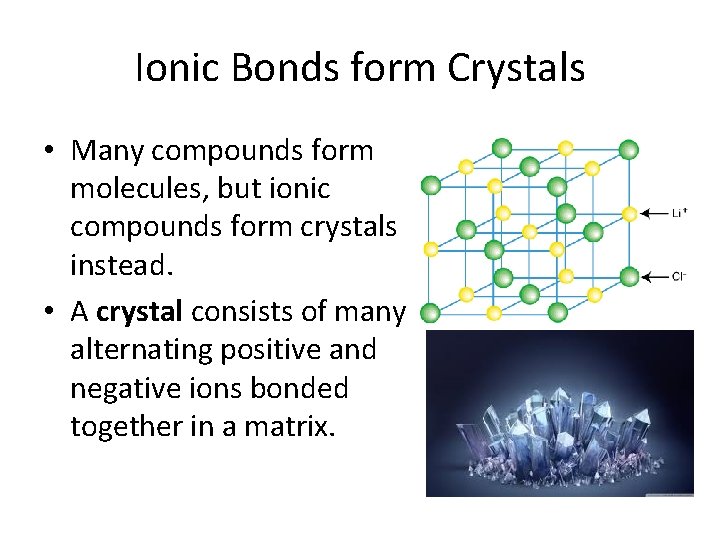
. Ions arrange themselves to attract the electrostatic attractive force between the ions. Ionic Compounds have high boiling and melting points as theyre very strong and require a lot of energy to break. It occurs when atoms share or transfer valence electrons.
It possesses polar and nonpolar characteristics. Explains the lattice structure of ionic crystals. Their atoms join together to form molecules crystals or other structures.
We have moved all content for this concept to for better organization. Ionic bonds are strong and the crystals are rigid. Ionic compounds form crystals.
Ionic bonds are atomic bonds created by the attraction of two differently charged ions. Chemistry 22062019 0520. Pure nonmetals form covalent crystals eg diamond as do covalent compounds eg zinc sulfide.
These compounds are brittle and break into small pieces easily. The atoms are held together by chemical bonds. Why do ionic compounds form crystals.
Examples of such crystals are the alkali halides which include. Ionic compounds form hard solids with flat faces and straight edges called crystals In a crystal the positive and negative ions are found repeating 3-d patterns which is called. The first reason is just due to size of the Ionic Compounds or the.
The bond is typically between a metal and a non-metal. The lattice that forms extends out in three dimensions. Click the link The reaction of sodium with chlorine and watch through the video there.
Briefly describe each of the steps involved with the reaction of sodium with chlorine. A simple cubic crystal lattice has ions equally spaced in 3D at 90 angles. It forms when atoms of a metal transfer electrons to atoms of a nonmetal.
Stability of ionic solids depends on lattice energy which is released in the form of heat when two ions are. Ionic solids are held together by the electrostatic attraction between the positive and negative ions. The elements in compounds are held together by chemical bonds.
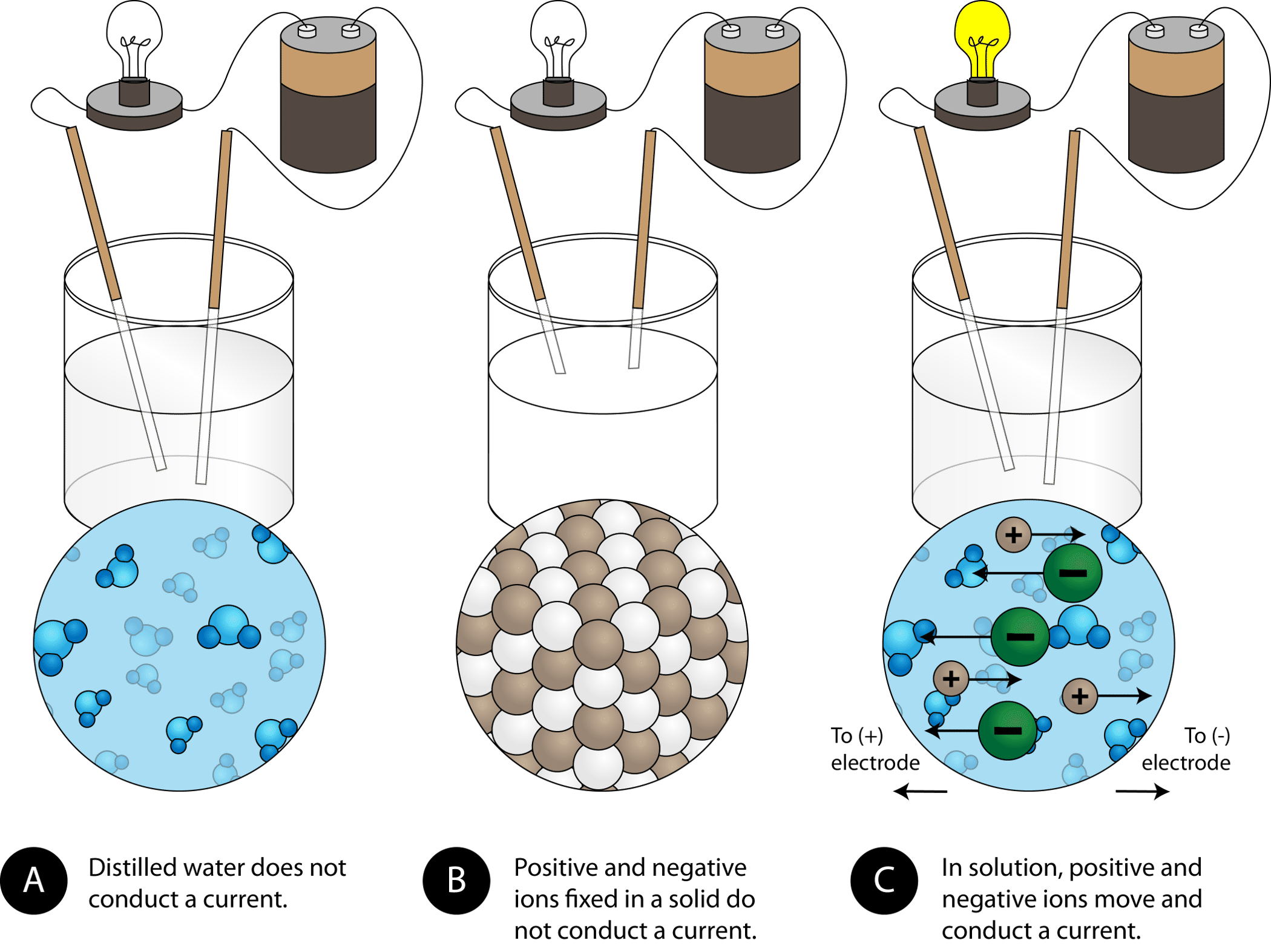
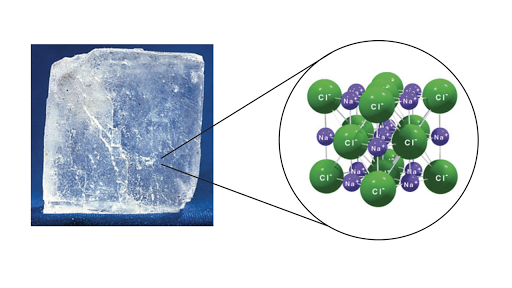
The electrostatic forces of attraction between oppositely charged ions lead to the formation of ions. The result is a three-dimensional structure of alternate Na and Cl ions. Ions bound together by electrostatic attraction form ionic crystals.
This is a crystal of sodium chloride. A chemical bond is a force of attraction between atoms or ions. The arrangement of ions in a regular geometric structure is called a crystal lattice.
When this happens the atoms become oppositely charged ions. Because there are repeated units crystals have recognizable structures. Why are ionic compounds good conductors of electricity.
Their arrangement varies depending on the ions sizes or the radius ratio the ratio of the radii of the positive to the negative ion. The crystal lattice shape is an arrangement that takes the least amount of energy to maintain. Ionic compounds form crystals instead of molecules.
In solid form ionic substances form crystal lattices with alternating positive and negative charged ions with a ratio and arrangement specific to the substance. Atoms in covalent crystals are linked by covalent bonds. A crystal is made up of an orderly and symmetrical pattern of atoms.
Ionic compounds form crystals to reduce the potential energy of the system being used. Explain why there is no such thing as a single molecule of salt. An ionic bond is the force of attraction that holds together oppositely charged ions.
Lonic compounds are electrical b. All of the following describes ionic compounds except. Briefly describe how an ionic bond forms.
Large crystals display flat regions faces and well-defined angles. A compound is a unique substance that forms when two or more elements combine chemically. Explore several common ionic crystal examples found in nature.
An ionic crystal consists of ions bound together by electrostatic attraction. Ionic crystals are crystalline structures that grow from ionic bonds and are held together by electrostatic attraction. They have high melting and boiling points d.
A chemical bond is a force of attraction between atoms or ions that share or transfer valence electronsFeb 19 2021. For example the sodium ions attract chloride ions and the chloride ion attracts sodium ions. Identify and describe the three ways that mutations affect organisms.
Look at the picture of the NaCl Crystal Schematic. A crystal consists of matter that is formed from an ordered arrangement of atoms molecules or ions. Compounds form as a result of chemical reactions.
How ionic compounds form crystals. All ionic crystals basically form the same shape. They are hard and brittle.
Kaypeeoh72z and 8 more users found this answer helpful. Elements form compounds when they combine chemically. When positive ions and negative ions are nearby they are attracted and pair up building an Ionic Crystal.
Please update your bookmarks accordingly. Ions arrange themselves in a three-dimensional array to maximize what type of force. Why do ionic compounds form crystals.
Ionic compounds form crystals to reduce the potential energy of the system being used. 2 Get Other questions on the subject. Entire molecules are bonded to each other in an organized manner.
What Are Ionic Crystals.

Ionic Compounds Ionic Bonds Properties Formation Examples Videos

Ionic Compound Ck 12 Foundation

Draw A Picture Of An Ionic Bond For Salt Nacl Ppt Video Online Download

Ii Ionic Compounds Salts E Properties I Form Crystalline Lattice Structures 1 Determined By X Ray Crystallography Ii Conduct Electricity When Melted Ppt Download

Chemical Bonding Ionic Compounds Ionic Compound 1 Ionic Compounds Form Crystals 2 High Melting And Boiling Points 3 Hard And Brittle 4 Conduct Electricity Ppt Download

Ionic Crystals Introduction To Chemistry

Ionic Compounds Noadswood Science Ppt Video Online Download

Ionic Bonds Essential Question How Do Ionic Bonds

Chapters 15 6 Ionic Bonding 15 1 Objectives Use The Periodic Table To Infer The Number Of Valence Electrons In An Atom And Draw Its Electron Dot Lewis Ppt Download

Ionic Crystals Introduction To Chemistry
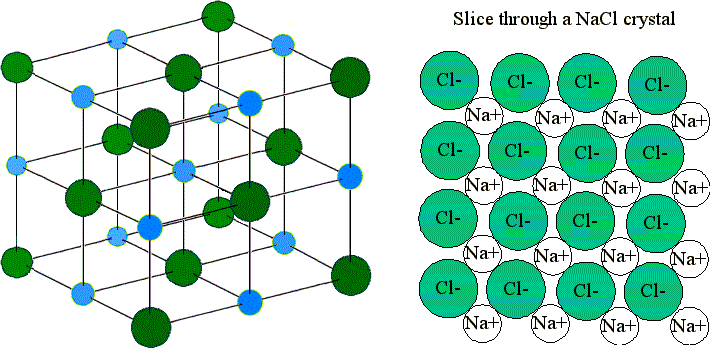
9 2 Ionic Bonding And Lattice Energy Chemistry Libretexts

Ionic Compound Ck 12 Foundation

Ionic Crystal Structure Ck 12 Foundation
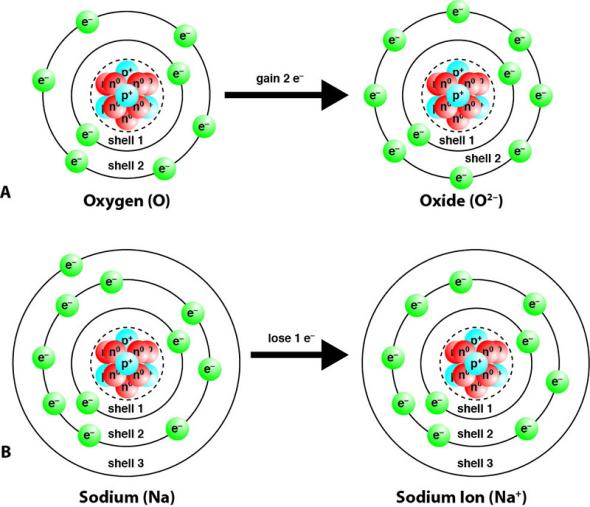
Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth

Physical Properties Of Ionic Compounds Ck 12 Foundation
Physical Properties Of Ionic Compounds Ck 12 Foundation
Molecules And Compounds Overview Atomic Structure Article Khan Academy


Comments
Post a Comment